Brain volume loss in relapsing multiple sclerosis: Indirect treatment comparisons of available disease-modifying therapies
Robert Zivadinov, et al. – University at Buffalo, SUNY.
NOTE: We are actively seeking user feedback on this preprint. To view the preprint and add commentary, go here.
Background: While numerous disease-modifying therapies (DMTs) are more effective than placebo in curbing relapses in MS clinical trials, data comparing the effectiveness of these DMTs are scarce. This is especially true for data on their effects in limiting disease progression.
This Study: Zivadinov and colleagues performed indirect treatment comparisons (ITCs) for 13 DMTs, using data on brain volume loss (BVL) from 28 clinical trials, most of which were Phase 3.
-
- Two approaches to ITCs were employed: a model-based meta-analysis (MBMA) and a network meta-analysis (NMA).
-
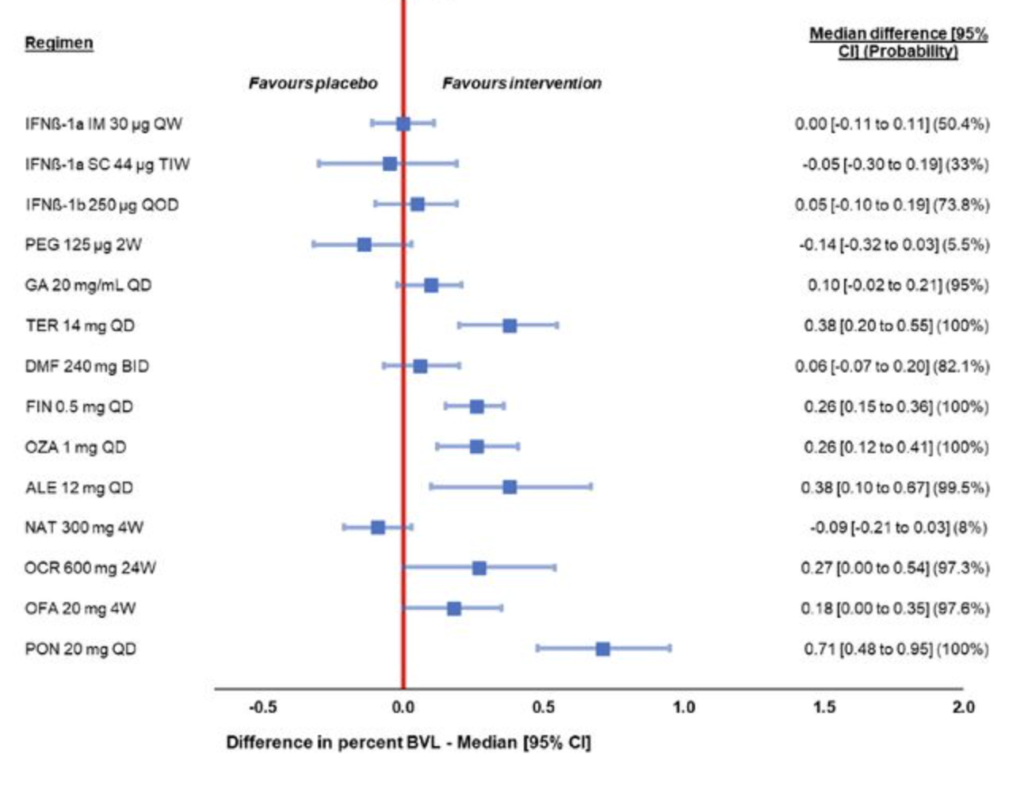
- Both approaches found the sphingosine-1-phosphate receptor subtype 1 (S1P1) modulator ponesimod to be far superior to all other DMTs examined, with a median difference (95% CI) in BVL of 0.71 (0.48-0.95) in the MBMA and 0.79 (0.48-1.10) in the NMA.
-
- The S1P1 modulator fingolimod was also found to be significantly more effective than placebo in both analyses, as was teriflunomide.
-
- Effects from alemtuzumab and the S1P1 modulator ozanimod were only significant in the MBMA, while effects from ofatumumab were only significant in the NMA.
Bottom Line: Four treatments demonstrated significant reductions in BVL for both models.
| Reduction in BVL v. Control (p<0.05) | ||
| DMT | MBMA | NMA |
| Ponesimod | 71% | 79% |
| Teriflunomide | 38% | 39% |
| Fingolimod | 26% | 45% |
| Ofatumumab | 18% | 27% |
Open Question: S1P1 modulators appear to be the most effective class of treatments; why is this? Is sequestering lymphocytes in lymph nodes the best way to limit their neurodegenerative potential, compared to targeting them with monoclonal antibodies? Could S1P1 modulators be affecting different, unexpected disease pathways to achieve their full effects?




