“Estradiol regulates local synthesis of synaptic proteome via sex-specific mechanisms.”
Pooja Raval, et al – King’s College London.
Background: Estradiol affects the composition of the synaptic proteome, but it is unclear whether this requires transcription of new mRNA and/or the translation of pre-existing mRNA.
This Study: Raval and colleagues examined the effects of estradiol on global and synaptic protein levels in mouse hippocampal slices of each sex and in mixed-sex primary hippocampal cultures.
- Estradiol’s impact on protein levels did not require transcription of new mRNA,indicating that estradiol induces translation of pre-existing mRNAs located in dendrites and spines.
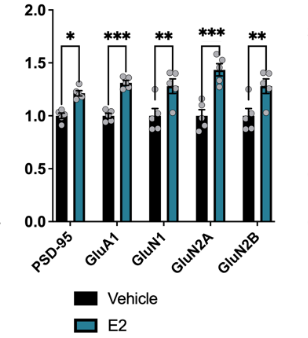
- Postsynaptic proteins upregulated by estradiol include the excitatory synaptic scaffolding protein PSD-95 and subunits of the excitatory NMDA receptor, which are critical for learning and memory.
- The mechanisms by which estradiol affects these proteins varies by sex. Blocking mammalian target of rapamycin (mTOR) signaling inhibited estradiol-driven increases of an NMDA receptor subunit in both sexes, but only inhibited increases of PSD-95 in male hippocampi.
- Notably, different sets of proteins associated with inhibitory synapses were downregulated by estradiol in each sex, suggesting that estradiol plays a broader sex-specific role in protein degradation as well as production.
Open Questions: How do changes in estradiol levels, across monthly cycles and over lifetimes, affect cognition and memory? This question was also raised by our featured preprint from last month examining the effects of estradiol and other menstrual cycle hormones on brain structure in naturally-cycling women.
Bottom Line: Estradiol influences synaptic proteins in a sex-specific manner on rapid time scales.




