Emil Gustavsson, et al. – University College London.
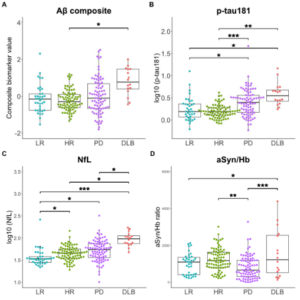
Background: The majority of the genetic risk for PD remains unexplained, indicating a need for uncovering additional risk factors. Rab GTPases are plausible candidates for their role in membrane trafficking and their interactions with known risk factor LRRK2.
This Study: Gustavsson and colleagues examined 130 families with multiple members affected by PD, a case-control series, and genomic databases for mutations in Rab GTPases.
- Three families had a point mutation to RAB32 resulting in a S71R substitution, with inheritance patterns indicating an autosomal dominant role in PD.
- While all affected members in these families had this mutation, several unaffected members also had the mutation, indicating incomplete penetrance as observed with other autosomal dominant genes in PD.
- The case-control and genomic data sets revealed 15 more people with PD bearing the RAB32 S71R mutation.
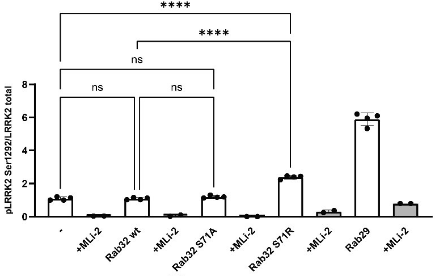
- Expression of the mutant RAB32 protein in HEK293 cells led to a ~2-fold increase in a marker of LRRK2 activation (phosphorylation at S1292) and a ~6-fold increase in phosphorylation of LRRK2 target RAB10.
Bottom Line: A mutation to RAB32 can cause PD in an autosomal dominant fashion, likely by increasing phosphorylation of LRRK2.
Open Question: Will examination of other cohorts, particularly with subjects of ancestries other than the European and North African ancestries represented in this study, find that mutations to other RAB GTPases can also cause PD?