“Plasticity of Human Microglia and Brain Perivascular Macrophages in Aging and Alzheimer’s Disease.”
Donghoon Lee, et al. – Icahn School of Medicine at Mount Sinai.
Background: Previous studies of human microglial subtypes in AD have yielded inconsistent results and failed to capture the full scope of subtype variation, likely due to small sample sizes and differences in sequencing technologies.
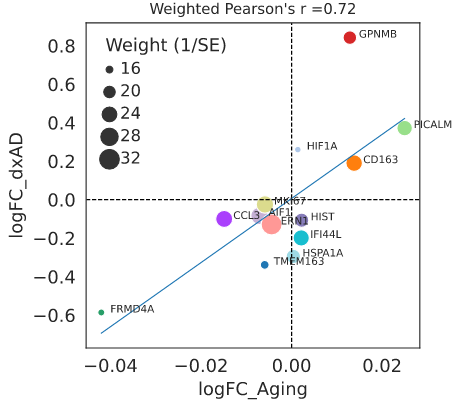
This study: Lee and colleagues profiled a total of 832,505 myeloid cells from 1,607 donors across 2 cohorts, identifying 13 subtypes. Comparing age-related changes in a dementia-free subset to AD-related changes in subjects ages 40 and older found that the proportions of all but one of these subtypes vary in similar ways across both conditions, suggesting that AD could represent an acceleration of the natural aging process. These changes include a shift in the balance of 2 homeostatic subtypes, with the FRMD4A subtype decreasing and the PICALM subtype increasing. Even the sole subtype to show a major difference, termed AD-associated microglia (ADAM), increased significantly with age, though not nearly to the same extent that it increased with AD severity. By identifying putative cell-cell interactions, they found the ADAM subtype to be a hub of cellular and molecular interactions.
Bottom Line: Mapping out changes in microglial subtypes across AD and aging found strikingly similar results, while also identifying a critical subtype that could drive pathological changes in AD and thus represent a therapeutic target.




