Betty M. Tijms, et al. – Vrije Universiteit Amsterdam.
In this study of CSF proteomes and genomes of patients with elevated Aβ42, patients with AD fell into 5 subcategories, with each representing a different affected biological pathway and pathology risk profile. These groupings could help assess the risk of AD in a given patient and indicate which treatments might work best for their specific risk factors.
Ramon Velasquez, et al. – Arizona State University.
Now published in Acta Neuropathologica.
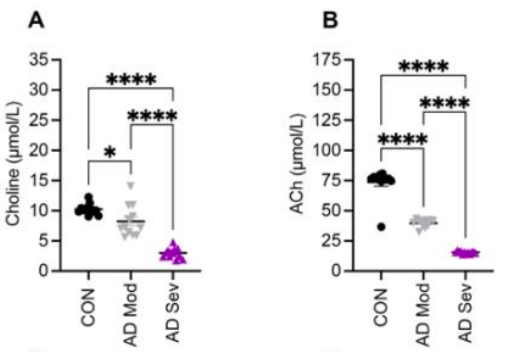
This study revealed that patients with AD show reduced levels of choline and several of its metabolites. Combined with their findings that choline supplementation improved Aβ and tau pathology in mouse models of AD, while choline deprivation worsened these measures, these results suggest that choline may mitigate AD pathology in patients at risk of AD.
Qiuyun Pan, et al. – Washington University School of Medicine.
Adapting a strategy developed by cancer immunologists, this study engineered macrophages that target amyloid plaques. These macrophages are capable of clearing plaques in mouse brain slices and in living mice, providing a proof-of-concept for their development as a potential therapeutic.
Pathological Tau transmission initiated by binding lymphocyte-activation gene 3. DCI: 2.0.
Chan Chen, et al. – Johns Hopkins School of Medicine.
In this study, Lag3 is identified as a receptor specific to Tau fibrils, but not monomeric Tau. Loss of Lag3 in vitro prevents the propagation of Tau pathology in mouse neurons, and loss in vivo alleviates anxiety and social cognition phenotypes associated with Tau fibrils.
Christopher Ramsden, et al. – National Institute of Aging, NIH Bayview.
This study of post-mortem sAD tissue finds that components of the ApoER2-Dab1 pathway are expressed in cell types that develop pTau during the early phases of the disease, suggesting a role for disruption of this pathway in sAD initiation. Note that in the Allen atlas of AD, SST+ interneurons are the only neuronal subtype to be positive for both ApoER2 and Reelin; the heightened vulnerability predicted by this model could explain why the Allen Institute found that SST+ neurons die very early in AD progression.
Additional notable preprints:
Inferring Alzheimer’s disease pathologic traits from clinical measures in living adults. DCI: 0.8.
Find a Zotero library with these preprints, as well as our Featured Preprints for June 2023, here.




