Microglial subtype that distinguishes AD from healthy aging?
Donghoon Lee, et al. – Icahn School of Medicine at Mount Sinai.
Lee et al. compared changes to microglial subtypes due to AD with those due to aging, identifying 1 subtype highly influenced by AD while finding the other 12 showed similar changes for both factors.
A protective variant with lower synapse loss, but more plaques
Ryan J. Bevan, et al. – Cardiff University.
This study examined the effects of the rare protective R522 variant of Phospholipase C Gamma 2 (PLCG2) relative to the common P522, finding lowered synapse loss in a mouse model of AD despite greater amyloid plaques.
Phase 2 AD drug blocks amyloid-β binding at synapses
Martí Colom-Cadena, et al. – University of Edinburgh.
Colom-Cadena and colleagues found that in brain tissue from patients with AD, amyloid-β (Aβ) binds to TMEM97 at synapses.
Navigation in mobile game stratifies players by AD risk and disease status
Uzu Lim, et al. – University of Oxford.
Sea Hero Quest (SHQ), a mobile game designed to provide new methods for identifying and tracking AD, distinguishes patients with AD from controls, as well as at-risk carriers of APOE4 from non-carriers.
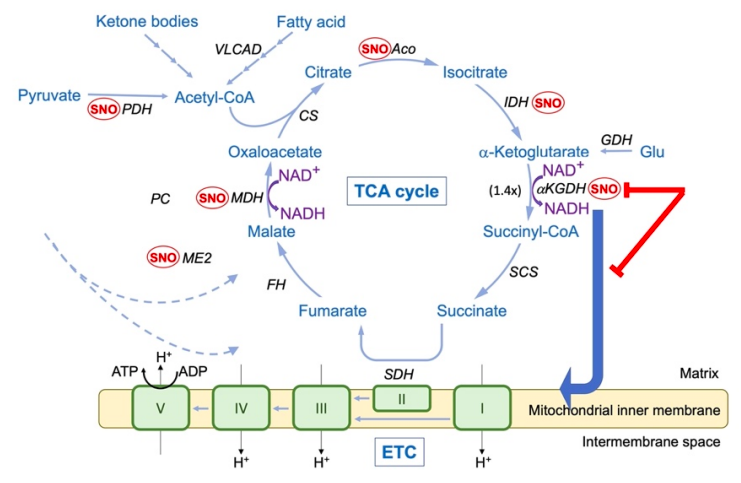
Bypassing metabolic block in AD neurons reduces synapse loss
Alexander Y. Andreyev, et al. – Scripps Research Institute.
Andreyev et al found that in patients with AD, key enzymes in the tricarboxylic acid (TCA) cycle of energy production are inhibited by S-nitrosylation, a post-translational modification where nitric oxide (NO.) binds to a cysteine residue.
Eliminating mitochondrial calcium uptake blocks AD progression in mouse model
Pooja Jadiya, et al. – Temple University.
By knocking out the mitochondrial calcium uniporter (Mcu) from forebrain neurons, Jadiya and colleagues were able to prevent memory deficits in a mouse model of AD, as well as reduce production of amyloid plaques, tau tangles, free radicals, and peroxidized lipids.
AD risk genes accelerate brain aging in healthy adults
James M. Roe, et al. – University of Oslo.
Examining data from a cohort of healthy (non-AD) adults assessed over decades, Roe and colleagues found that subjects with elevated polygenic risk scores for AD (PRS) experienced greater declines in volume of the hippocampus, particularly left hippocampus, and left amygdala than other subjects of the same age.




