Johan Larsson, et al. – Linköping University.
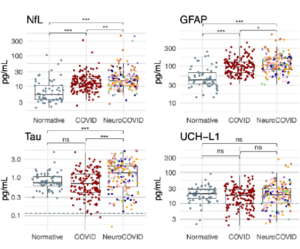
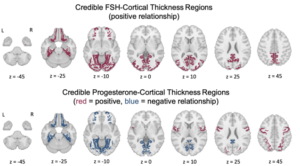
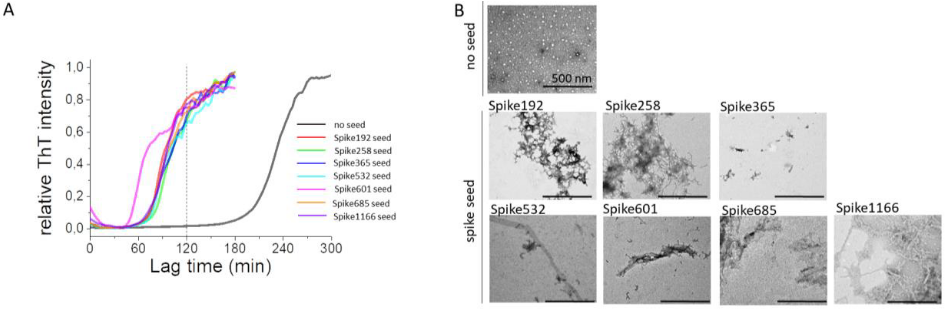
Larsson and colleagues report that amyloidogenic fragments of the SARS-CoV-2 (COVID) spike protein can serve as seeds for amyloid-β 1-42 (Aβ42) fibril formation. This cross-reactivity is specific to spike protein fragments, as amyloids of other proteins could not serve as seeds. Spike protein fragments also served as seeds for human prion protein (HuPrP), which causes Creutzfeldt-Jakob Disease (CJD) when misfolded, with different fragments serving as the most efficient seeds for Aβ42 and HuPrP. Given the neurological impact of “long COVID” and apparent links between COVID infection and Alzheimer’s/CJD, the ability for SARS-CoV-2 to induce neurodegenerative amyloids would pose a significant public health concern. Moreover, since other human-targeting viruses, such as influenza, also have amyloidogenic proteins, cross-seeding of endogenous proteins like amyloid-β could contribute to links between viral infection and neurodegenerative disorders, such as how the shingles vaccine lowered dementia risk by nearly 20%.