Biomarkers seemed to be everywhere at CMSC. Presenters discussed the great need for clinical assays that can provide a more objective measurement of MS disease status and progression and laid out the strengths and weaknesses of candidate fluid and imaging biomarkers.
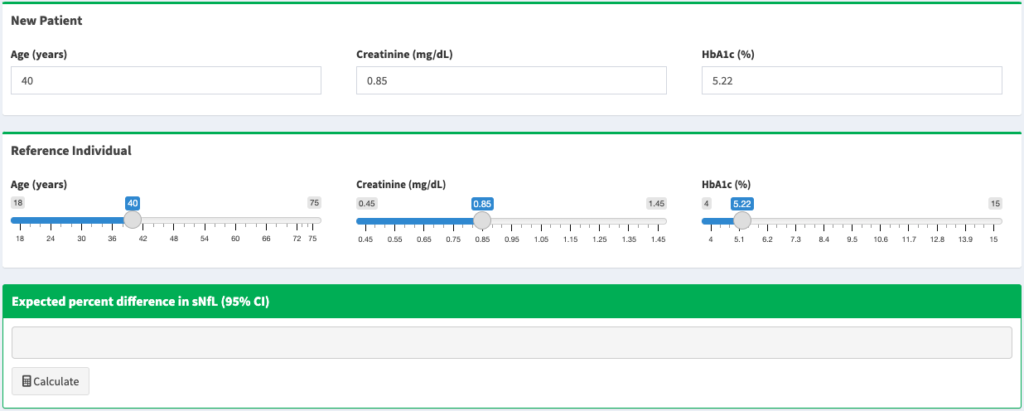
- Neither of the two most prominent serum biomarkers, neurofilament-light (NfL), a marker of myelin degeneration, nor glial fibrillary acidic protein (GFAP), a marker of reactive astrogliosis, are particularly sensitive or specific as markers of severity or risk of progression. Data presented by Gauruv Bose suggested that the two biomarkers together provided a far better predictor of worsening disability than either taken individually. He also showed that NfL is generally a better biomarker than GFAP. Peter Calabresi shared data from the MS PATHS network demonstrating that NfL measurements depend heavily on individual covariates such as age, BMI, race, smoking and comorbidity such as diabetes and hypertension. He shared a link to a website created by Kate Fitzgerald that calculates expected NfL levels based on individual patient characteristics. As a final caveat, Sharmilee Gnanapavan showed data demonstrating significant variability in repeated measurements of NfL in the same individual.
- Octave Biosciences discussed clinical uses for their MS Diagnostic Assessment (MSDA), an assay measuring levels of 18 proteins in patient blood samples to provide a Disease Activity Score and 4 Disease Pathway Scores. Augmenting measurements of NfL, GFAP, and MOG with these additional 15 proteins yields a detailed assessment of a patient’s disease state to better inform treatment decisions.
- To determine which treatments are most likely to succeed in a specific patient, Dr. Amit Bar-Or described the “Immune Health” fingerprinting approach being developed by his team at the University of Pennsylvania. This combines RNA sequencing and epitope assessments to identify which immune cells are dysfunctional and how they have been altered; this allows for tailoring treatment to the unique needs of each patient, rather than a “one size fits all” plan.
- In addition to these molecular approaches, Dr. Mike Wattjes described a number of recent advances in imaging-based biomarkers. These include detection of lesions with paramagnetic rim to assess chronic active lesions, as well as the use of TSPO as a PET tracer for measuring activated macrophages/microglia and astrocytes.
- To use spinal cord atrophy as a marker of neurological damage, Dr. Blake Dewey of Johns Hopkins described advances in machine learning that allow for gaining the spatial resolution of advanced 3D imaging with more conventional 2D images. In his model, named SMORE, the high-resolution plane being directly imaged (e.g. axial) is blurred to simulate the quality of the low-resolution planes (e.g. sagittal and coronal). The model learns how to process the artificially-blurred plane into the ground-truth high-res plane, then applies the training to the other two planes to gain a high-quality 3D image. Because the model is trained for each new set of images using that specific set, there is no risk of differential bias based on the choice of initial training data. This technology will make acquiring high-quality images of patients easier, cheaper, and more widely available.
Further reading:
Find a Zotero library with this manuscript here.




