Antara Rao, et al. – UC San Francisco.
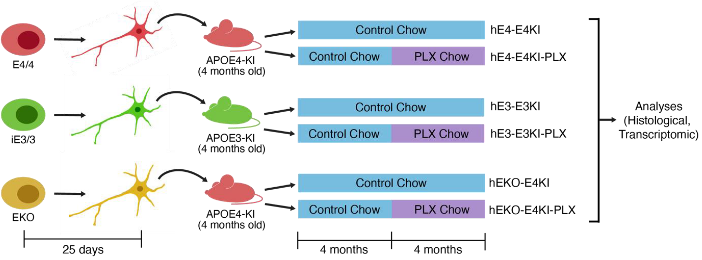
Background: While astrocytes are the main source of APOE in the brain under typical conditions, neurons and microglia can also produce it under stressful conditions. The relative impact of APOE, and particularly APOE4, from these cell types in AD pathology is unclear.
This Study: Rao and colleagues extended prior work with a novel chimeric mouse model they developed to explore the effects of neuronal vs microglial APOE4. Their previous study found that transplanting human neurons derived from induced pluripotent stem cells (hIPSC neurons) that express APOE4 into the hippocampi of mice expressing human APOE4 (E4KI) leads to the formation of amyloid-β plaques; this is in the absence of the familial AD mutations required for plaque formation in other models, making it a better model of late-onset AD. Here, they compared transplanted E4KI mice with APOE3 mice transplanted with APOE3 hIPSC neurons (E3KI) and APOE4 mice transplanted with hIPSC neurons carrying non-functional APOE alleles (hEKO-E4KI). Additionally, they gave half of each group chow with PLX3397 (PLX), which depletes microglia, to examine the relative contribution of microglia. Depleting the microglia from E4KI mice reduced the number of amyloid-β plaques and tau aggregates, indicating a role for microglia in these aspects of APOE4-mediated AD pathology. While hEKO-E4KI mice showed even greater numbers of amyloid-β plaques than E4KI mice, they had drastically lower levels of tau aggregates, and the majority of the amyloid-β plaques are the diffuse variety observed in cognitively normal elderly subjects, rather than the dense-core plaques seen in AD and that are predominant in E4KI mice.
Bottom Line: Microglia and neuron-derived APOE4 are jointly required for promoting amyloid-β and tau aggregates, while neuronal APOE4 is specifically required for the formation of dense amyloid-β plaques.




