Juan Codocedo, et al. – Indiana University School of Medicine.
Background: The function of immune cells throughout the body, including microglia, is tied to their metabolic state. Recent studies have drawn opposing conclusions about the role of hexokinase-2 (HK2), which phosphorylates glucose to place it in the glycolysis pathway, in regulating inflammatory microglia.
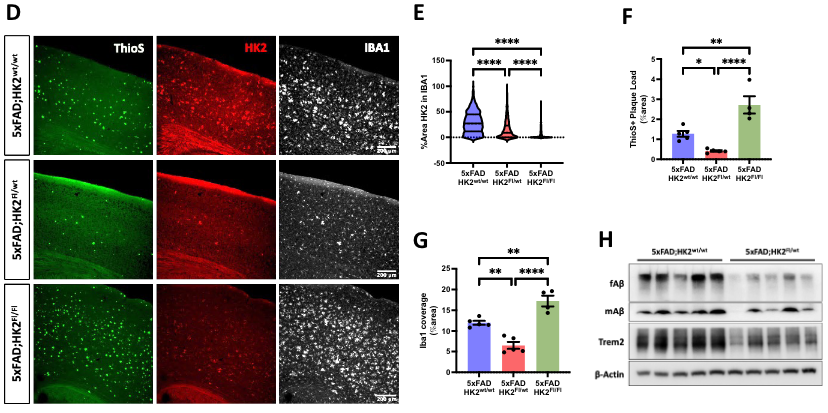
This Study: Cococedo and colleagues examined the effects of HK2 in 5xFAD model mice in three ways: deleting one copy of HK2 from microglia (fl/WT), deleting both copies from microglia (fl/fl), and orally administering the anti-cancer HK2 inhibitor lonidamine (LND). Deleting one copy of HK2 reduced amyloid plaque burden, reduced numerous markers of inflammatory microglia, and improved cognition as measured by alternations on a Y-maze. Deleting both copies, however, either erased these benefits or led to worse outcomes relative to controls. LND produced benefits similar to those seen after deleting one copy of HK2, but only in male mice. Given that in vitro administration of LND produced similar anti-inflammatory benefits in microglia derived from mice of either sex and female mice had higher brain concentrations of LND than male mice provided the same weight-adjusted dose, these sex differences could be due to greater drug concentration in the brains of female mice causing excessive HK2 inhibition. Rather than affecting mitochondria or metabolism, the benefits of partial HK2 inhibition may be due to a mechanism first observed in cancer cells where HK2 triggers degradation of IKBα, which leads to nuclear translocation of the transcription factor NF-κB and the expression of pro-inflammatory genes. Treating microglia cultures with LND increased IKBα levels and blocked the ability of amyloid-β to trigger NF-kB translocation to the nucleus.
Bottom Line: Metabolic enzymes can directly regulate the inflammatory state of murine microglia. Administering existing HK2 inhibitors, at doses that provide the correct level of inhibition after accounting for sex differences, could represent a new approach for treatment.




