Kenji Tagai, et al. – National Institutes for Quantum Science and Technology.
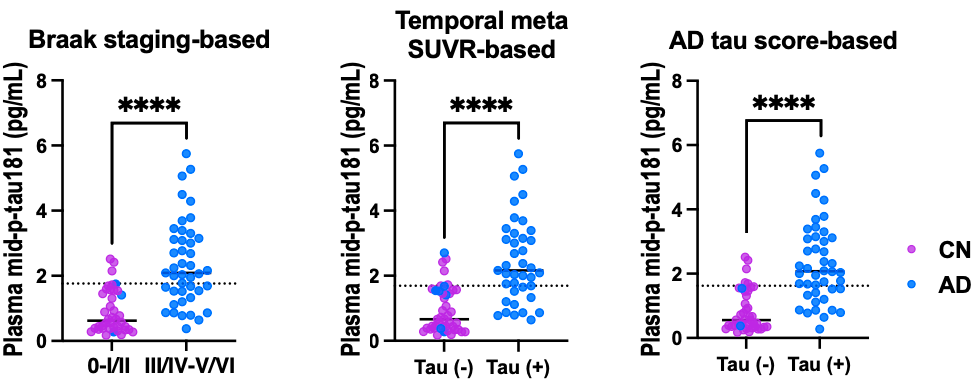
This study reports a novel immunoassay that uses plasma biomarkers to measure tau aggregation. Other pTau assays mainly correlate with amyloid, not tau, and use antibodies targeting the N-terminal region along with phospho-specific antibodies. By targeting the midsection of the protein, this assay captures tau that has been cleaved at both the N- and C-termini, which better reflects the tau species that form tau aggregates.
The AUC for distinguishing subjects by tau positivity, depending on which method of tau-PET interpretation was used, ranges from 0.855 to 0.870. The assay is quite specific (88.1%-92.5%), though the sensitivity is less than ideal (62.8%-71.8%). These values could be improved by a two-cutoff system, a scheme becoming widely used with other biomarkers, where patients with intermediate scores receive tau-PET for more definitive analysis. Using phosphorylation at residues other than p-Tau181, such as p-Tau217, may also yield a more conclusive assay. Nevertheless, this represents a major advancement in the ability to measure tau buildup in a minimally invasive, scalable manner.




