Early indications from CMSC suggest we are on the verge of major new options for MS treatment. Two signaling molecules, Bruton’s tyrosine kinase (BTK) and CD40 ligand (CD40L), are the targets of two classes of DMTs working their way through clinical trials and may define the potential next generation of patient care in MS.
Existing DMTs.
The objective of most current DMTs is to prevent B-cell entry into the CNS; ocrelizumab and other anti-CD20s do so by depleting B-cells, while fingolimod prevents lymphocytes from leaving lymph nodes. These approaches, however, do little to affect the B-cells that already reside within the CNS, as anti-CD20 antibodies do not enter the CNS. Strategies focused on depleting B-cells, or keeping them sequestered to lymph nodes, have broader impacts on immune system function that come with their own risks. Furthermore, approaches that target B-cells will not necessarily prevent harm from microglia, which drive much of the progressive damage in MS.
How are BTK inhibitors different?
As small molecules that can traverse the BBB, BTK inhibitors are capable of targeting the CNS-resident immune system. Once there, they target B-cell antigen-related pathways, both in terms of antigen-induced maturation and the presentation of antigens to T-cells. In microglia, blocking BTK reduces expression of pro-inflammatory genes and promotes expression of those that return these cells to a homeostatic profile. Beyond these functions, BTK inhibitors may also be acting as anti-EBV agents. Gavin Giovannoni noted during his presentation of this year’s Whitaker Lecture that EBV uses BTK to promote the survival of infected B-cells during the latent phase of infection. This would suggest that the most critical effect of BTK inhibition is preventing the survival of specific EBV-laden B-cells, as opposed to the maturation of B-cells in general.
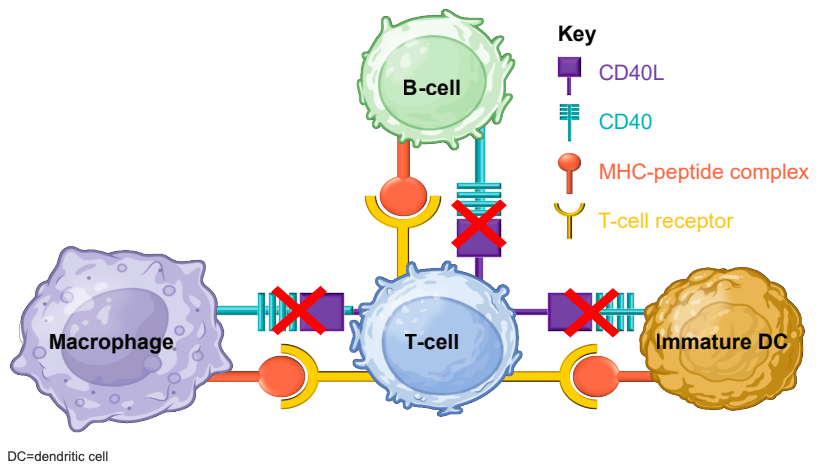
How are anti-CD40Ls different?
Antibodies raised against CD40L block the pathway used by B-cells to co-stimulate T-cells and monocytes; this co-stimulation is required for these cells to mount responses to antigens. Since CD40 expression is upregulated in patients with MS, anti-CD40L therapies can potentially bring this signaling pathway back to appropriate levels. As for CD40L, Giovannoni cited studies finding that EBV drives B-cells to express CD40L to promote host cell transformation and survival, suggesting that blocking CD40L may reduce survival of virus-laden B-cells. Given recent studies finding that EBV infection is all but a prerequisite for developing MS (see our post about EBV for more details), a role for these therapies in blocking EBV pathology should not be ignored.
Promising clinical data.
Whatever their critical target(s) may be, clinical trial data for drugs targeting these pathways are promising. One BTK inhibitor, evobrutinib, significantly reduced new Gd+ lesions and slowly evolving lesions (SELs) in a dose-dependent manner during a Phase 2b trial, according to Amit Bar-Or’s presentation. The effects on SELs, as well as the biology of BTK, are a large part of what make BTK inhibitors a potential game-changer – a DMT that can curb the progressive damage of MS, not just control relapses. As for the anti-CD40L antibody frexalimab, late-breaking Phase 2 clinical data presented by Gavin Giovannoni showed that 44 of 46 participants on high doses of the frexalimab were free of new Gd+ T1 lesions by Week 24. This was accompanied by a lack of severe or fatal TEAEs, as well as a lack of treatment-emergent SAEs. View the poster presenting these data on frexalimab here.
With Phase 3 trials for the BTK inhibitor tolebrutinib completing in August and September of this year, the future of MS care may be arriving quite soon.
Further reading:
Find a Zotero library with these manuscripts here.




