“Disease-associated astrocyte epigenetic memory promotes CNS pathology.”
Hong-Gyun Lee, et al. – Harvard Medical School.
Background: Exposure to inflammatory signals leaves circulating immune cells primed to mount a larger response to subsequent inflammatory signals. It is unclear whether astrocytes, which also respond to inflammatory signaling, possess a similar epigenetic memory.
This Study:
- Lee and colleagues found that astrocytes in mice given the inflammatory cytokines IL-1β and TNF mounted a stronger response when these cytokines were administered 7 days later.
- Analogous in vitro tests found that the chromatin for pro-inflammatory genes was more accessible, priming astrocytes to exhibit a larger response.
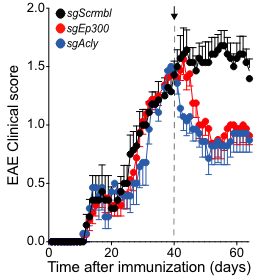
- Inactivating Ep300 and Acly, which promote epigenetic chromatin opening, prior to induction of an experimental autoimmune encephalitis (EAE) model of MS reduced symptom intensity and chromatin accessibility of pro-inflammatory genes. .
- Staining tissue samples from patients with MS found that primed astrocytes are almost exclusively found in chronic lesions, especially chronic active lesions, suggesting a role in the smoldering inflammation that drives disease progression.
- Inactivating Ep300 or Acly in the NOD EAE model, which more closely resembles SPMS, 40 days after EAE induction significantly improved symptoms.
Bottom Line: In both mouse models and patients, astrocytes can become primed to deliver more intense inflammatory responses, suggesting a role for these primed astrocytes in disease progression and a potential therapeutic target.
Open Question: Could pharmacological inhibitors of ACLY be future candidates for MS treatments? These inhibitors are currently being evaluated for treating cancer; testing them in the context of MS may yield new options for patients.




